© 2003 Khusnul
Yaqin
Posted 22 September, 2003
Science
Philosophy (PPs 702)
Graduate
Program / S3
Institut
Pertanian Bogor
September 2003
Instructors:
Prof Dr Ir Rudy C
Tarumingkeng (Principal)
Prof Dr Ir Zahrial
Coto
Can artificial sea grass
mimics natural sea grass?
by
Khusnul Yaqin
P062030151
E-mail: kyaqini@plasa.com
Introduction
Sea grass plays important role in coastal habitat.
The presence of sea grasses may increase the abundance of organisms by
increasing (i) the amount of physical structure usable as living space;(ii) the
number of microhabitats; (iii) sediment deposition and stabilization;(iv) food
resources and (v) protection from predators. Sea grass may also reduce
hydrodynamic forces. The ability of sea grass beds to fulfill the majority of
these roles is well documented, through both biological (e.g. food resources)
and physical (e.g. protection offered by canopy structure). (Lee, et al.,
2001).
Despite the importance of sea grass in the
coastal zone is well recognized, declines in sea grass meadows are global
(Walker and McComb, 1992). These declines may result from natural events such
as ‘wasting disease’ (Den Hartog, 1987) or high-energy storms (Patriquin,
1972). Most sea grasses loss, however, have resulted from human activities such
as eutrophication (Buithuis and Woelkerling, 1983; Cambridge and McComb, 1984;
Neverauskas, 1987), sedimentation (Kirkman, 1978; Buithuis et al., 1984; Lee, 1997), land reclamation and changes in land use (Kemp et
al., 1983).
Rehabilitation by using
artificial sea grass is one of possibility method to recover destroyed sea
grass bed in coastal habitat. As rehabilitation instrument the artificial sea
grass has to have some similarities to natural sea grass in order to substitute
the role of natural sea grass in the marine environment. In fact, it is
impossible that artificial sea grass able to substitute natural sea grass
completely. However, up to certain point artificial sea grass fill in existence
of natural sea grass. Therefore, it can act as natural sea grass does.
Artificial sea grass is usually used in
ecological and behavioral sea grass experiment as imitating or collecting
instrument. This instrument is also often used in field experiments to
investigate the relationship between associated fauna and the physical
structure of the sea grass.
The aim of this essay is to briefly discuss
at what extent an artificial sea grass can mimic natural sea grass.
Artificial sea grass
Many types of artificial sea grass are used as research or rehabilitation device. In principle, the construction is simulated similar with natural sea grass, which is consisting of leaf, erect shoot and rhizome. One of example of this construction is the laboratory studies instrument that are using wooden dowels, strips of plastic, rubber bands and a 66-foot-long flume to make model beds that mimic different types of natural sea grass and conditions (figure 1.).
rhizome root erect
shoot
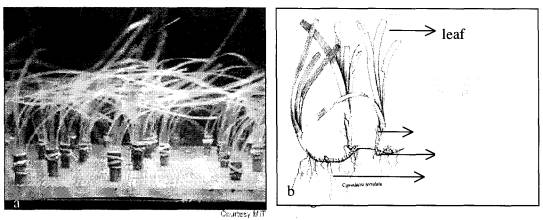
Fig. 1. Comparison between artificial sea grass
construction and natural sea grass morphology. (a) Artificial sea grass
construction as laboratory study instrument (Nept, 1999). (b) Natural sea grass
morphology, Cymodocea serrulata (Anonym, 1997).
Physical Aspects
The construction of artificial sea grass is created for
imitating the function of natural sea grass in water column. Physically,
artificial sea grass can imitate what natural sea grass act as a baffle for the
incoming water flow, which decrease the current velocities and allow deposition
surround it. Almasi et al. (1987) studied the effect of
natural and artificial sea grass on sedimentation rates. They observed that the
mean flow velocity in the grass-free area was higher than in the sea grass
area. Consequently, the increase depositional rate of sediment within the sea
grass was due to slowing of water current by the sea grass blades. Bostrom and
Bonsdorff (2000) demonstrated that density-dependent effect of Zotera-like and
Ruppia-like of artificial sea grass not only
act as current-baffling, particle-trapping and sediment-binding, but also
minimize possibility of in fauna dislodgement. In addition, Kenyon et al.
(1999) observed that fine detritus particles were visibly accumulated on
artificial sea grass with 24 h of deployment. A gentle environment due to these
actions probably produce biological advantages for many types of marine biota
namely, sheltering, feeding and nursery ground and epiphyte substrate.
Biological
Aspects
As food resources natural sea grass leaves play unimportant role. Only few animals appear directly to use sea grass production as food; some fish, turtles, sirenians and a few sea urchins with ruminant-like cultures of cellulose-splitting bacteria in theirs
guts are notable exception. Bamess and Hughes (1999) estimated only 5%
of sea grass production is consumed directly.
One of food resources that play important
role is epiphyte, which attaches on sea grass blades. Artificial sea grass
blades that made from strapping band, which have rough surface, are suitable
substrate for epiphyte. Yaqin (1998) found that composition of the epiphyte
contain 42.2% Bacillariophyceae, 15.8% Cyanophyceae, 15.8% Chromonadea,
10.2% Sarcodina, 5.8% ciliata, and 3.6% Crustacea. Fifteen species of
epifauna were recorded by Lee et al.(2001) in the experimental
artificial sea grass; nine gastropods, five brachyurans and one bivalve.
Epiphytic structure reveals different composition
and abundant between artificial and natural sea grass (Pinckney and Micheli,
1997). Lee, et al. (2001) found Amphipods, microgastropods and
polychaetes were common in the Zostera bed, but not in the artificial
sea grass. This difference is probably a result of the differences in
biological quality between artificial sea grass and Z. japonica. The presence of living rhizomes and spatially complex root system, the
ability to release organic carbon (Penhale and Smith, 1977 in Lee et
al. 2001) and influence of nutrient fluxes (McRoy and McMilland, 1977 in
Lee et al., 2001) by natural sea grass may be contribute to difference in
the faunal community between Artificial and natural sea grass.
Epiphytic structure appeared to play only a
limited role in determine the density of most mobile fauna, but epiphytic
structure appeared to be important in augmenting the settlement of bivalves
(Bologna, et al., 1999). These structure increased microstructure on the
artificial sea grass blades and might influence settlement by the adhesiveness
of the surface. Bologna et al. (1999) found that when epiphytes were abundant on artificial sea grass
blades, the densities of small grazers (primarily crustaceans and gastropods)
were significantly greater.
Artificial sea grass not only provides
epiphyte as food resources, but also provides eligible sheltered habitat for
numerous marine fauna. It is because artificial sea grass attract physically a
fauna similar to that of natural sea grass (Bell et al., 1985; Virnstein
and Curran, 1986;Sogard, 1980 in Sogard and Able, 1994). Liu and
Lonerangan (1996) found that large postlarvae (2.7mm CL) and juvenile (4.1 mm
CL) of tiger prawn (Penaeus
semisulcatus and P. esculentus) both were more abundant on artificial sea
grass than bare sand during the day but not at night, which was indicating that
they used it as sheltered habitat.
The development of a dial pattern in
immigration of marine fauna to artificial sea grass habitat could linked to
variability of behavior and/or predation risk. The more active and larger
individual colonized artificial sea grass at night and vice versa (Sogard and
Able, 1994). Bauer (1985) suggested that reduced predation risk from
visual hunters at night allowed caridean shrimp to move up into water column
above the sea grass canopy. Similarly, Vance (1992 in Sogard and Able,
1994) proposed that a pattern of higher nighttime activity in three species of
penaeids resident in sea grass and mangrove habitat was related to decrease fish
and bird predation at night.
Chemical Aspects
Interaction between artificial sea grass blades and the epiphytes create both sediment and contaminant trap. Nept (1999) suggest that sometimes the artificial canopy “captures” contaminants by creating still regions that allow particulate material to accumulate around stems and leaves. In addition, this canopy create little of turbulence, which may improve the uptake of elements like phosphorous and nitrogen, or may accelerate the uptake of chemicals by microbial communities living on the artificial blade surfaces.
Liu and Lonerangan (1996)
observed that both larvae and juveniles of Penaaeus semisulcatus were greater numbers on natural than artificial sea grass during the day.
Juvenile P semisulcatus were also more abundant on natural than
artificial sea grass. This difference is probably due to chemical compounds
that produced by natural sea grass. Study on lobster postlarvae (Homarus americantis) indicates that they can detect sea grass
extract in seawater and use them for orientation (Boudreu et al. 1993 in
Liu and Lonerangan, 1996). The chemical cue emitted from the sea grass
might influence the habitat preference of lobster post larvae (Boudreu et al.,
1990, 1993, in Liu and Lonerangan 1996). The presence of eelgrass
accelerates the molt time of blue crab megalopae (Forward et al., 1994 in Liu
and Lonerangan 1996) and it was suggested that eelgrass provide both chemical
and natural cue to megalopae. Therefore, it was indicated that chemical cue
play important role in biotic interaction which is not occur in artificial sea
grass.
Conclusion
It is unavoidable that non-living thing
cannot imitate living thing completely. In the case artificial sea grass, it
may be able to mimic physical aspect of natural sea grass almost perfectly such
as water movement baffling, particle trapping and sediment binding. Although,
it depends on how similar the artificial sea grass construction to natural sea
grass. On the other hand, in term of chemical and biological aspects,
artificial sea grass can imitate fewer aspect of natural sea grass than those
on physical aspects. It is because there is no biotic interaction between
marine fauna and artificial sea grass.
References
Almasi, M.N. Hoskin C.M, Reed J.K, and Milo,
J. 1987. Effects of natural and artificial Thalassia on rates of sedimentation.
J. Sedimentary Petrology. Vol. 57, pp. 901-906.
Anonim, 1997. What is sea grass http://www.bayconnect.com.aulsea- grass/sea grass htm/top.
Barnes, R.S.K. and R.N. Hughes. 1999. An
introduction to Marine ecology. Blackwell science Ltd.Oxford. London.
Bauer, R.T.
1985. Dial and seasonal variation
in species composition and abundance of caridean shrimp (Crustacea, Decapoda)
from seagrass meadow on north coast of PuertoRico. Bulletin of Marine Science
36;150-162.
Bologna, P.A.X. and K.L. Heck Jr. 1999.
Macrofaunal associations with seagrass epiphytes relative importance of tropic
and structural characteristics. J. Exp. Mar. Biol. Ecol., Vol. 242, pp. 21-39.
Bostrom, C. and Erik B. 2000. Zoobenthic
community establishment and habitat complexity — the
importance of sea grass shoot-density, morphology and physical disturbance for
fauna! recruitment. J. Mar. Ecol.Prog. Vol.205, pp. 123-138.
Bulthuis, D.A. and Woelkerling, W.J. 1983.
Biomass accumulation and shading effects of epiphytes on the leaves of the sea
grass, Heterozostera tasmanica in Victoria, Australia. Aquatic Botany
16:
137-
148.
Bulthuis, D.A., Brand, G.W. and Mobley. M.L. 1984.
Suspended sediments and nutrients in water ebbing in from sea grass-covered and
denuded tidal mudflats in a southern Australian embayment. Aquatic Botany 20:
257-266.
Cambridge, ML and McComb. A.J. 1984. The loss
of sea grasses in Cockburn Sound, Western Australia. I. The time course and
magnitude of sea grass decline in relation to industrial development. Aquatic
Botany 20: 229-243.
Den Hartog. C. 1987. ‘Wasting disease’ and
other dynamic phenomena in Zostera beds. Aquatic Botany 27: 3-14.
Kemp, W.M., Boynton, W.R., Twiggy, R.R.,
Stevenson, C. and Means, J.C. 1983. The decline of submerged vascular plants in
Upper Chesapeake Bay: Summary of results concerning possible causes. Marine
Technology Society. Journal 17: 78-89.
Kenyon, R.A., M.D.E. 1-laywnod, D.S. Heales,
N.R. Lorengan, R.C. Pendrey and D.J.. Vance. 1999. Abundance of Fish and
Crustaceans postlarvae on portable artificial seäg.~.s~1ii1iits: daily sampling
provides quantitive estimates of the settlement of new recruits. J. Exp.
Mar. Biol. Ecol., Vol. 232, pp. 197-216.
Kirlunan, H. 1978. Decline of sea grass in
northern areas of Moreton Bay, Queensland. Aquatic Botany 5: 63-76.
Lee, S.Y., C.W. Fong, R.S.S. Wu, 2001. The
effects of sea grass (Zosterajaponica) canopy structure on associated fauna: a
study using artificial sea grass units and sampling of natural beds. J. Exp.
Mar. Biol. Ecol., Vol. 259, pp. 23-50.
Liu, H.,
N.R. Lonerangan, 1996. Size and
time of day affact the response of postlarvae and early juvenile grooved tiger
prawns Penaeus semisulcatus De Haan (Decapoda: Penaeidae) to natural and
artificial seagrass in the laboratory.J. Exp. Mar. Biol. Ecol., Vol. 211,
pp. 263-277.
Nept (1999). Researchers strive forperfectwetland.http://www.enn.com/enn-news-archive/i
999/05/050399/perfect_2975.asp.
Neverauskas, V.P. 1987. Monitoring sea grass
beds around a sewage sludge outfall in South Australia. Marine Pollution
Bulletin 18: 158-164.
Patriquin,
D.G. 1972. Carbonate mud
production by epibionts on Thalassia: an estimate based on leaf growth rate
data. Journal of Sedimentary Petrology 42: 687-689.
Pinckney, J.L. and F. Micheli. 1997.
Microalgae on seagrass mimic:Does ephiphyte community structure differ from
live seagrass?. J. Exp. Mar. Biol. And Ecol. Vol. 221, pp. 59-70.
Sogard, S.M. and K.W. Able, 1994. Diel
Variation in Immigration of Fishes and Decapod Crustaceans to Artificial sea
grass habitat Estuary Journal Vol 17 pp 622 630
Walker, DI. and McComb, A.J. 1992. Sea grass
degradation in Australian coastal waters. Marine Pollution Bulletin 25: 191-195.
Yaqin, K. 1998. Penerapan lamun buatan sebagai cara rehabilitasi padang lamun
alami. Laporan hasil penelitian. Fakultas Ilmu Kelautan dan Perikanan
Universitas Hasanuddin, Makassar.